In Hungary there are several research groups settled in universities and research institutes or companies working on the fields of capillary electrophoresis and flow analysis. The leaders of the largest these groups are the following: Prof. András Guttman, MTA-PE Translational Glycomics, University Pannonia, Veszprém; Prof. Ferenc Kilár, Bioanalysis Institute, University of Pécs; Prof. Attila Gáspár, Department of Inorganic and Analytical Chemistry, University of Debrecen; Prof. Éva Szökő, Department of Pharmacodynamics, Semmelweis University, Budapest. Capillary electrophoresis is widely applied in a few companies (e.g., Gedeon Richter Plc., Budapest).
The overall goal of the group of Prof. András Guttman is to develop and apply new integrated technologies for the deep glycomics analysis of circulating tumor cells from human and animal samples and specifically isolate and target biomedically relevant glycoproteins from hundreds to a few thousand cells for diagnostic and therapeutic applications. There are many important studies that are not conducted where samples are only available at this low level. The major goals to address present day challenges in glycomics technology were sampling, sample handling, development of ultra-sensitive microfluidics based separation techniques in conjunction with mass spectrometry and novel glycoinformatics tools. The sampling/sample preparation part includes new approaches for trapping circulating tumor cells by immuno- and other affinities onto microfabricated nano-structures (e.g., pillars, nanotubes, etc.), followed by lysing the cells, glycoprotein capture, enzymatic release of the glycans, fluorophore labeling and separation / analysis, all with a new generation of chemically modified surfaces to minimize sample losses at such low sample levels. The microfluidic sample preparation system is coupled to capillary electrophoresis (CE) or capillary electrochromatography (CEC) with ultra-high separation power and detection sensitivity via ESI-LIF-MS.
The goals of Prof. Attila Gáspár’s group to perform a complex studies of pharmaceuticals (solution stability, pK, logP, pharmacokinetical parameters, etc.) and to determine pharmaceuticals and their metabolics in clinical samples. These investigations can be performed with the various techniques of capillary electrophoresis (CZE, MEKC, CIEF, CGE) more quickly than other alternative techniques. Using microfabrication procedures they design and prepare microfluidic chips (lab-on-a-chips) in the size comparable with computer chips using soft lithography. In their research chromatographic, electrophoretic and electrochromatographic separation systems are integrated into the chips made from a flexible, transparent plastic polydimethylsiloxane (PDMS). The LC, CE or CEC separation of pharmaceuticals can be done in the same chip in a multiplex channel system. Recently, they study the designing and fabrication of microfluidic devices with high specific volume and their analytical applications. The developed microchips are applied for extraction and immobilized enzyme reactor and their hyphenation with sensitive detectors.
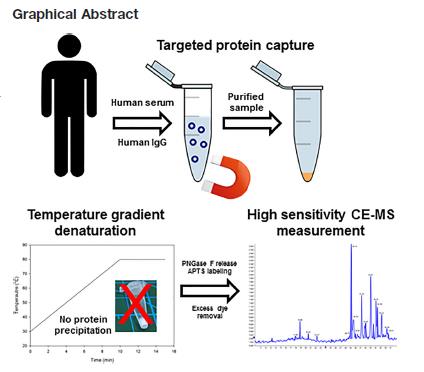
Figure 1. Efficient sample preparation workflow for deep N-glycomics analysis from serum
Brief (hi)story of Flow injection analysis and Capillary electrophoresis in the Czech Republic (former Czechoslovakia)
Petr Chocholouš1, Miroslav Polášek1, Petr Kubáň2, František Foret2
1Department of Analytical Chemistry, Faculty of Pharmacy in Hradec Kralové, Charles University, Czech Republic
2Institute of Analytical Chemistry of the Czech Academy of Sciences, Veveří 97, 602 00, Brno, Czech Republic
Flow Injection Analysis
Flow Injection Analysis (FIA) was invented in 1975 by Jarda Růžička (Czechoslovak) and Elo H. Hansen at the Technical University of Denmark [1]. The research on this innovative technique in Czechoslovakia begun in the early '80s at the Department of Analytical Chemistry, Faculty of Pharmacy in Hradec Králové, Charles University. Their previous experience with spectrophotometric flow-through measurements made it only a tiny step to construct the FIA manifold and adapt it to automate reagent-based assays. The lack of sophisticated and expensive instrumentation available at this period in Czechoslovakia favored a simple FIA setup. Several papers were published in national journals from the middle of the '80s, followed by the first paper published internationally in Analytica Chimica Acta in 1989 [2] describing the system for determining urea in body fluids utilizing an immobilized urease reactor made of Control Porous Glass sorbent.
At this early period, the biggest challenge was constructing a device, which would allow the injection of precise volumes of sample into the flowing stream. After many trials, the manually operated valve allowed precise injection of two solutions within the range of 20 to 200 microliters simultaneously when fabricated in a faculty workshop. The designing and fabricating of peristaltic pumps, mixing connectors, and reaction coils completed the FIA analyzer (Fig. 1). The setup was recognized as a significant advance of then-existing technology and became a model for serial production. Fortunately, an unusual candidate was found in the Unified Agricultural Cooperative "1st of May" in the village of Pouchov, near Hradec Kralove. This FIA 20 Analyzer, furnished with SPEKOL photometer (manufactured in East Germany), and chart recorder, became a milestone in accepting FIA in our country. It spread in agrochemical and industrial laboratories thanks to the tutorials done in the Faculty of Pharmacy [3]. During the late '80s, many reagent-based assay protocols, including chloride, ammonia, nitrite, nitrate, phenols, boron, and molybdenum in soils, water, and plant materials, were developed. FIA protocols for calcium, proteins and other clinical analytes became available too. The key contribution to the beginnings and development of FIA in this time was by Prof. Rolf Karlíček, Assoc. Prof. Miroslav Polášek a Prof. Petr Solich.
After the fall of the Iron curtain in 1989, we could travel abroad and widely present our research at international meetings and in international journals, where our work became gradually recognized. At the 9th International Conference on Flow Injection Analysis conference in Orlando in 1997, we established a close collaboration with the founders of FIA and Sequential Injection Analysis (SIA) – Jarda Růžička, Elo H. Hansen, and Garry Christian. Then we were entrusted with the organization of the 10th ICFIA in Prague in 1999. In 2008, we organized the first international SIA conference in Hradec Králové, then Flow Analysis conference in 2015 in Prague, and together with Jagiellonian University in Kraków, Poland, the FA&CE 2018 conference in Hradec Králové.
At the turn of the millennium, thanks to younger scientists, the research expanded with newer SIA technique - H. Sklenářová [4,5]; monolithic column employing medium-pressure liquid chromatography technique named Sequential Injection Chromatography (SIC) – P. Solich, D. Šatínský and P. Chocholouš [6]; and a syringe pump as a reaction/extraction chamber based Lab-in-syringe – B. Horstkotte [7]. Close cooperation with companies producing flow systems (FIAlab and GlobalFIA, both USA) promoted significant developments in the pumps and flow systems designs. Many scientists dealing with Flow analysis and related techniques, from Austria, Sweden, Slovakia, Ukraine, Russia, Portugal, Spain, Germany, Argentina, Brazil, Japan, Australia, and namely Jarda Růžička as Fulbright Professor in 2008, cooperated and visited Czech laboratories. A significant number of automated methods for SPE, including testing nanofibers as SPE sorbents [8], SIC [9], various miniaturized LLE methods [10] including Lab-in-syringe technique, and even single-drop extraction in fully automated mode[11]. The sample preparation methods were online coupled to HPLC [12], GC [13], or ICP [14]. The other way of the research is monitoring kinetic profiles of the liberation of active substances from novel pharmaceutical formulations [15] or real-time monitoring of interactions between active/toxic substances and cell membrane transporters [16]. A long list of published articles in renowned ISI journals is followed by several national patents and hosting of the online tutorial on Flow Injection Analysis written by Jarda Růžička [17].
In the last 25 years, other groups in the Czech Republic were dealing with the development of Flow analysis, located at the Faculty of Natural Science in Prague, Charles University.
Milestones in the development of Flow Analysis in the Czech Republic
1985 - lab-made FIA
1998 – FIA-SPE
2001 – SIA chemiluminescence
2002 – lab-made SIA (and software)
2002 – SPE-SIA
2002 – SIC (Sequential Injection Chromatography)
2003 – SIA based drug liberation apparatus
2009 – 1st generation of commercial SIC
2010 – DLLME (dispersive liquid-liquid microextraction) dual valve SIA
2015 – 2D SIC
2016 – SIA based monitoring of drug permeation across a cell monolayer
2018 – 2nd generation of commercial SIC
2018 – LIS GC (Lab-in-syringe with Gas Chromatography)
2019 – LIS HPLC (Lab-in-syringe with Liquid Chromatography)
2019 – SIA + 3D printed analyzer parts
Fig.1:

Capillary electrophoresis
Electrophoretic analytical methods have a long history, starting from the basic theoretical work by Kohlrausch [18] in 1897, throughout the beginning of the last century, witnessing the work of Tiselius on the mobilities of weak electrolytes [19] and the work of Hjerten [20] and Virtanen [21] that have set the basic concepts of the electromigration techniques, such as isotachophoresis and capillary electrophoresis.
Historically, in former Czechoslovakia, electrophoretic methods such as analytical electrophoresis and isotachophoresis have a long tradition and were studied since 1960-1970s. Around 1970, the first commercial isotachophoretic instrument from the Swedish company LKB was available and this instrument could be used with certain modifications also for electrophoresis in PTFE capillaries. It has been a lucky coincidence that the researchers at the scientific institutions of former Czechoslovakia had very good contacts with the University of Eindhoven in the Netherlands and one of the pioneers of ITP and CE, Prof. F.M. Everaerts. Several institutions, most notably the Charles University, the Institute of Analytical Chemistry of the Czechoslovak Academy of Sciences in Brno and Comenius University in Bratislava were all involved in the pioneering work on electrophoresis. Probably one of the first publications is the joint publication of Prof. Jiri Vacik and F.M. Everaerts on the use of countercurrent in isotachophoresis [22]. One of the other co-authors of this publication was Jiri Zuska, who has become later famous for his work on the construction of contactless conductivity detectors [23], (together with Prof. Bohuslav Gaš from Charles University in Prague). Inspired by the work of Everaerts, Prof. Petr Boček and his colleagues at the Institute of Analytical Chemistry in Brno have contributed significantly to the development of theory and methodology of modern electrophoresis. Prof. Dušan Kanianský at the Comenius University in Bratislava has also contributed to the progress of this newly developing technique, among others by initiating the production of a new commercial instrument for analytical isotachophoresis in Spisska Nova Ves in Slovakia. The company, now Villa Labeco [24], has now a near 30 year history of a successful production of various electrophoretic instruments. Other researchers, such as Prof. Zdeněk Stránský [25] from Palacký University in Olomouc or Prof. Vladimír Jokl [26] from Faculty of Pharmacy in Hradec Králové, Charles University and their coworkers have made a significant contribution to the theoretical (Jokl's equation of ion mobility) and practical capillary electrophoresis. The historical milestones in the development of isotachophoresis and capillary electrophoresis in the Czech Republic/Czechoslovakia were recently reviewed [27,28], both articles are unfortunately in Czech only.
Since the development and pioneering work of the scientists in Prague, Brno, and Bratislava, electrophoretic analytical techniques have gained their steady place in nearly all laboratories in the Czech Republic. To name only a few prominent institutions that have significantly contributed to the development of electrophoresis, we must mention the work of Prof. Bohuslav (Bob) Gaš at the Charles University, who took over the research group after Prof. Vacík in 1996. He is the author of the famous electrophoretic simulation software PeakMaster [29] and Simul [30] that allow computer simulations of electrophoretic separations to be performed. Bob Gaš was also one of the co-authors of the two-electrode contactless conductivity detector [31]. The detector was until recently produced by the deceased electrical engineer Jiří Zuska (2020) and his company Admet. The credit in the late development of various methods using the C4D detector by Jiří Zuska goes also to Petr Tůma at Charles University, who applies it for medical research.
Without any doubt, the Institute of Analytical Chemistry in Brno is the most famous for its contribution to the development and propagation of electrophoretic techniques. The theoretical works of Prof. Petr Boček and Dr. Petr Gebauer have explained many phenomena occurring during electrophoretic separations. One of the most cited papers is the one on the separation of anions in drinking water from 1983 [32]. Later the development of a CE sample splitter by Deml et al. [33] or the use of absorbing co-ion in indirect UV detection [34] are among their prominent research pieces that are worth mentioning. Another significant contribution to the instrumental application of electrophoresis and its coupling with mass spectrometry was accomplished by the current director of the Institute, Dr. František Foret, who spent significant time at Barnett Institute in Boston, USA and continued his research on CE-MS in Brno. There has been also interest towards practical/clinical applications of CE, lead by younger scientists, Dr. Pavel Kubáň, Assoc. Prof. Petr Kubáň, Dr. Jana Křenková and other colleagues at the Departments of Electromigration methods and Bioanalytical instrumentation.
Other institutions and researchers in the Czech Republic that are actively working with capillary electrophoresis are Prof. Vaclav Kašička at the Institute of the Organic Chemistry and Biochemistry in Prague, engaged in the development of theory, methodology, and instrumentation of capillary electromigration (CE) methods and their application for the separation, analysis, micropreparation, and physico-chemical characterization of (bio)molecules, the researchers at Palacký University in Olomouc (Profs. Lemr, Ševčík; Assoc. Profs. Bednář, Barták, Petr) who work with studies of electrochemical conversion, ionization in mass spectrometric measurements or online preconcentration in capillary electrophoresis and coupling of CE to ICP-MS in analyses of biological samples, forensic evidence and archaeological samples, and Assoc Prof. Pospíšilová with Dr. Urbánek working on the coupling of ITP and CZE.
Many members of the research teams are Editorial board members of prominent analytical journals (F. Foret, B. Gaš, V. Kašička) and an international conference CECE (Central European Capillary Electrophoresis), is organized on an annual basis in Brno.
An overlap
It is not a coincidence that Flow analysis methods and Capillary electrophoresis are discussed at the same time in this article. A combination of automated flow sample treatment schemes with separation techniques like CE can generally enhance the analytical power of both analytical techniques. This was recognized more than 20 years ago by Petr Kubáň and Bo Karlberg at Stockholm University, where the first article on the coupling of flow analysis and capillary electrophoresis was published [35]. At approximately the same time, Prof. Z.L. Fang [36] in China presented the same concept. Since then, different approaches and devices have been developed, including the combination of sequential injection analysis to CE (SIA-CE) [37] by one of the pioneers of flow injection analysis, Jarda Růžička. The research shows that these two techniques have much more in common than it may seem at first glance, and their "marriage " was certainly the happy one.
The whole article is a compilation of many stories of our colleagues from the past, present, and hopefully the bright future of these methods. Many thanks to all of them!
REFERENCES
[1] Růžička J., Hansen E.H.: Analytica Chimica Acta 78(1), 145 (1975).
[2] Solich P., Polášek M., Karlíček R., Valentová O., Marek M.: Analytica Chimica Acta 218, 151 (1989).
[3] National standard for determination of nitrogen in water - ČSN EN ISO 11 732, Jakost vod - Stanovení amoniakálního dusíku průtokovou analýzou (CFA a FIA) a spektrofotometrickou detekcí, Český normalizační institut (1998).
[4] Solich P., Svoboda A., Sklenářová H., Polášek M., Karlíček R.: Instrumentation Science and Technology, 30, 13 (2002).
[5] Sklenářová H., Svoboda A., Solich P., Polášek M., Karlícek R.: Instrumentation Science and Technology, 30, 353 (2002).
[6] Šatínský D., Solich P., Chocholouš P.,Karlíček R.: Analytica Chimica Acta 499, 205 (2003).
[7] Maya F., Horstkotte B., Estela J.M., Cerdà V.: Analytical and Bioanalytical Chemistry 404, 909 (2012).
[8] Šrámková I.H., Carbonell-Rozas L., Horstkotte B., Háková M., Erben J., Chvojka J., Švec F., Solich P., García-Campaña A.M., Šatínský D.: Talanta 197, 517 (2019).
[9] Chocholouš P., Vacková J., Šrámková I., Šatínský D.,Solich P.: Talanta 103, 221 (2013).
[10] Andruch V., Acebal C.C., Škrlíková J., Sklenářová H., Solich P., Balogh I.S., Billes F., Kocúrová L.: Microchemical Journal100, 77 (2012).
[11] Šrámková I., Horstkotte B., Sklenářová H., Solich P., Kolev S.D.: Analytica Chimica Acta 934, 132 (2016).
[12] Fikarová K., Cocovi-Solberg D.J., Rosende M., Horstkotte B., Sklenářová H., Miró M.: Journal of Chromatography A 1602, 160 (2019).
[13] Horstkotte B., Lopez de los Mozos Atochero N., Solich P.: Journal of Chromatography A 1555, 1 (2018).
[14] Sánchez R., Horstkotte B., Fikarová K., Sklenářová H., Maestre S., Miró M., Todolí J.-L.: Analytical Chemistry 89, 3787 (2017).
[15] Klimundová J., Šatínský D., Sklenářová H., Solich P.: Talanta 69, 730 (2006).
[16] Sklenářová H., Rosecká M., Horstkotte B., Pávek P., Miró M., Solich P.: Analytica Chimica Acta 1153, 338296 (2021).
[17] https://www.flowinjectiontutorial.com/, accessed Apr 30, 2021
[18] Kohlrausch F.: Ann. Phys. Chem., N. F. 62, 209 (1897).
[19] Tiselius A.: Nova Acta Regiae Soc. Sci. Uppsaliensis 7, 1 (1930).
[20] Hjertén S.: Arkiv Kemi 13,151 (1958).
[21] Virtanen R., Kivalo P.: Suomen Kemistilehti B, 182 (1969).
[22] Everaerts F. M., Vacík J., Verheggen Th. P. E. M., Zuska J.: J. Chromatogr. 60, 397 (1971).
[23] Vacík, J., Zuska, J., Muselasova, I.: J. Chromatogr. 320, 233 (1985).
[24] https://www.villalabeco.sk/, accessed Apr 30, 2021
[25] Stránský Z.: J. Chromatogr. 320, 219 (1985).
[26] Jokl V., Polášek M., Pospíchalová J.: J. Chromatogr. 391, 427 (1987).
[27] Křivánková, L.: Chem. Listy 114, 10 (2020).
[28] Gebauer, P., Foret, F.: Chem. Listy 114, 3 (2020).
[29] https://web.natur.cuni.cz/gas/peakmaster.html, accessed Apr 30, 2021
[30] https://web.natur.cuni.cz/gas/simul.html, accessed Apr 30, 2021
[31] Gaš, B., Zuska, J., Coufal, P., van de Goor, T., Electrophoresis 23, 3520, 2002.
[32] Gebauer P., Deml M., Boček P., Janák J.: J. Chromatogr. 267, 455 (1983).
[33] Deml M., Foret F., Boček P.: J. Chromatogr. 320, 159 (1985).
[34] Foret F., Fanali S., Ossicini L., Boček P.: J. Chroma-togr. 470, 299 (1989).
[35] Kubáň, P., Engstrom, A., Olsson, J.C.,Thorsen, G., Tryzell, R., Karlberg, B.: Anal. Chim. Acta 337, 117 (1997).
[36] Fang, Z.L., Liu, Z.S., Shen, Q.: Anal. Chim. Acta 346, 135 (1997).
[37] Wu, C.-H., Scampavia, L.. Ruzicka, J.: Analyst 127, 898 (2002).
Flow analysis and Capillary electrophoresis in Slovakia
Flow Injection Analysis
Research in the field of miniaturized and automated analytical methods is one of the key topics addressed by the research team at the Department of Analytical Chemistry of the Institute of Chemical Sciences, Faculty of Science, Pavol Jozef Šafárik University in Košice. The scientific aim of the research group is the development of procedures that are in line with the principles of green analytical chemistry and thus ensure the originality of the proposed procedures and schemes that could be applied to the determination of analytes in various types of samples.
Team of Dr. Šandrejová and Prof. Andruch is focused on using the flow systems, namely sequential injection system for automation and miniaturization of analytical procedures. The aim of this investigation is to develop commercially available flow systems as new, universal systems for application of new procedures for the analysis of plant materials, pharmaceuticals, biological and environmental samples. There are many original studies, for example connection or use of sequential injection technique for extraction procedures such as dispersive liquid-liquid microextraction [1,2], salting-out homogeneous liquid-liquid extraction [3], ultrasound-assisted surfactant-mediated extraction [4] using new green solvents [5] or non-extraction processes automated by SI system [6,7].
Prof. Basel and coworkers just recently started with the development of new "hand-made" and fully automated systems with an Optical Immersion Probe (OIP). These systems are characterized as easily available, inexpensive, easy and efficient to use in analytical chemistry. The interconnection of flow systems with OIP has proven to be very convenient because it can speed up the measurement process, minimize the amount of reagents used, and mainly can significantly improve the precision of the measurements. These systems can ensure not only the precise determination of analytes but also can improve the precision of the estimated parameters like rate constants, acid-base properties, or molar extinction coefficients [8]. The precise establishing of these characteristics with using the automated system can facilitate access to the analytical capabilities of various reagents and expand the scope of their use in analytical chemistry.
The development of flow analysis in Košice followed up on previous cooperation with colleagues from the Czech Republic, which also resulted in joint scientific publications [9-11], as well as with colleagues from Poland [12,13], which evolved into participation in the upcoming V4 Symposium "Flow Analysis and Capillary Electrophoresis" (FACE 2020).
In the flow analysis in Slovakia, it is also necessary to mention the area of flow electroanalytical methods, which is addressed by the group of Prof. Ernest Beinrohr at Slovak University of Technology in Bratislava. They deal with automation of electrochemical methods, e.g., [14,15].
Capillary Electrophoresis
In the recent years, capillary electrophoresis techniques have undergone significant development and evolution. The most commonly used electrophoretic techniques include zone electrophoresis and isotachophoresis. One of the current challenges in the field of analytical instrumentation for electrophoresis is the development of microchip electrophoresis. This technique can integrate all functions of a modern analytical laboratory, i.e., sample preparation, separation and detection, or also post-chip sample handling (lab-on-a-chip). The concept of lab-on-a-chip is currently very attractive in analytical and bioanalytical sciences.
In Slovakia, a founder of the scientific school in the field of electromigration separation methods was Prof. Dušan Kaniansky at the Comenius University in Bratislava. He had significantly contributed to the development of capillary electrophoresis and microchip electrophoresis not only in Slovakia but also in other countries. His research was focused on the development of electrophoretic analyzers and multidimensional electroseparations and trace and ultratrace analysis of multicomponent mixtures of substances on the capillaries and microchips. The analyzers developed in his laboratory began to be manufactured in 1982 in the Institute of Radioecology and Applied Nuclear Techniques (now the company Villa Labeco) in Spišská Nová Ves [16]. During the period 1982–2010, more than 800 advanced analyzers were produced and used in clinical, environmental, water management and other workplaces. The work of Prof. Kaniansky and his coworkers, which introduced coupling of capillary isotachophoresis with radiometric [17] and amperometric detection [18] as well as mass spectrometry [19], holds a significant position in the early developments of capillary electrophoresis. Prof. Milan Hutta, an expert in the field of liquid chromatography and a very good friend and coworker of Prof. Kaniansky, also contributed to development of capillary electrophoresis by its coupling with liquid chromatography [20].
At present, the following research groups are engaged in the development of electroseparation methods in Slovakia:
A research group at the Department of Analytical Chemistry, Faculty of Natural Sciences, Comenius University in Bratislava, which continues in the work of Prof. Kaniansky, deals with both capillary electrophoresis and microchip electrophoresis. The main focus of this research group is development of microchip electrophoresis with various types of detection techniques (conductivity detection [21], Raman spectrometry (Fig. 1) [22], ion mobility spectrometry [23], etc.) for the analysis of complex biological, food and pharmaceutical samples.
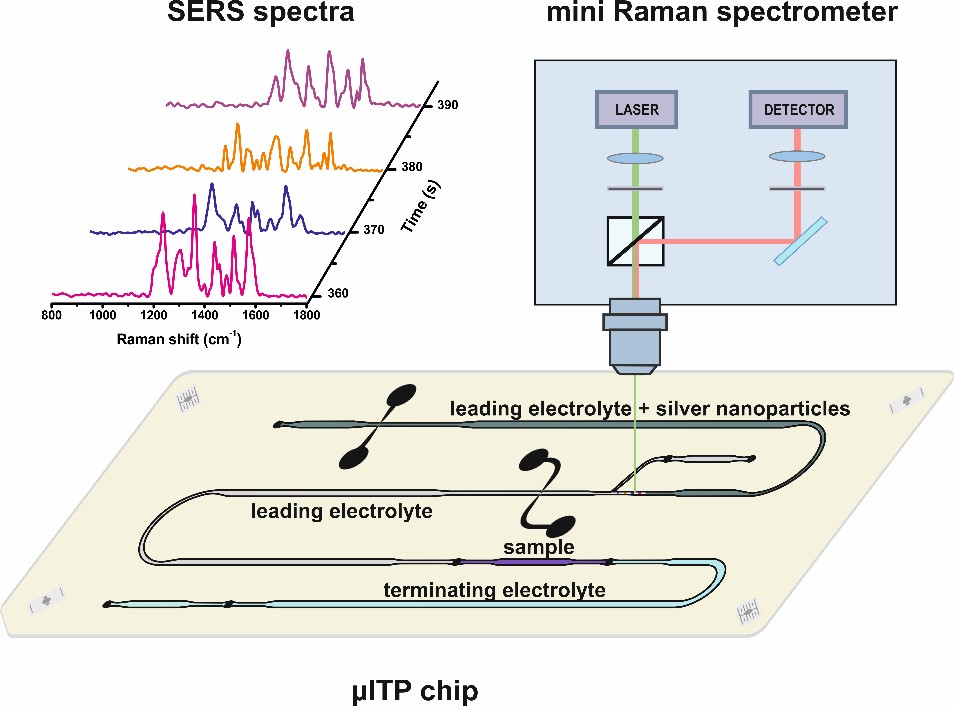
Fig. 1. Scheme of coupling of microchip electrophoresis with Raman spectroscopy.
A research group at the Department of Pharmaceutical Analysis and Nuclear Pharmacy, Faculty of Pharmacy, Comenius University in Bratislava, deals mainly with the development of electrophoretic separation methods for the determination of pharmaceuticals and biologically active substances in biological samples [24].
In common laboratory practice, capillary electrophoresis is well established for the diagnosis of various diseases [25], for the determination of drugs in complex samples [26] or for the separation of nucleic acids or proteins [27]. Production of capillary electrophoresis analyzers in Slovakia is handled by company Villa Labeco. These analyzers are used in the environmental and clinical laboratories not only in Slovakia, but also in Czech Republic, Poland, Hungary, Germany, etc. The application sheets developed by the company are focused on the determination of various inorganic and organic ions in water, soil, and food samples by capillary zone electrophoresis, isotachophoresis, or by combining different electrophoretic techniques [28]. Some of the application sheets are used, e.g., in the National Water Reference Laboratory for Slovakia.
The authors of this article, J. Šandrejová from the Department of Analytical Chemistry, Institute of Chemical Sciences, Faculty of Science, Pavol Jozef Šafárik University in Košice (Flow Injection Analysis), and M. Masár and J. Hradski from the Department of Analytical Chemistry, Faculty of Natural Sciences, Comenius University in Bratislava (Capillary Electrophoresis) want to express their thanks to all of the colleagues and teachers, and at the same time, they apologize to those, who are not included in this brief history and current status of flow analysis and capillary electrophoresis in Slovakia. All mentioned publications, and many others, are results of the work of enthusiastic groups of people from all over the world, who are always keen to learn and look for new research opportunities.
References
[1] V. Andruch, C.C. Acebal, J. Škrlíková, H. Sklenářová, P. Solich, I.S. Balogh, F. Billes, L. Kocúrová, Automated on-line dispersive liquid-liquid microextraction based on a sequential injection system, Microchem. J. 100 (2012) 77-82.
[2] M. Alexovič, V. Andruch, I.S. Balogh, J. Šandrejová, A single-valve sequential injection manifold (SV-SIA) for automation of air-assisted liquid-phase microextraction: Stopped flow spectrophotometric determination of chromium(VI), Anal. Methods 5 (2013) 2497-2502.
[3] M. Falkova, M. Alexovič, M. Pushina, A. Bulatov, L. Moskvin, V. Andruch, Fully automated on-line flow-batch based ultrasound-assisted surfactant-mediated extraction and determination of anthraquinones in medicinal plants, Microchem. J. 116 (2014) 98-106.
[4] A. Pochivalov, Ch. Vakh, V. Andruch, L. Moskvin, A. Bulatov, Automated alkaline-induced salting-out homogeneous liquid-liquid extraction coupled with in-line organic-phase detection by an optical probe for the determination of diclofenac, Talanta 169 (2017) 156-162.
[5] L. Nugbienyo, A. Shishov, S. Garmonov, L. Moskvin, V. Andruch, A. Bulatov, Flow method based on liquid-liquid extraction using deep eutectic solvent for the spectrofluorimetric determination of procainamide in human saliva, Talanta 168 (2017) 307-312.
[6] Y. Bazel, A. Tupys, Y. Ostapiuk, O. Tymoshuk, J. Imrich, J. Šandrejová, A simple non-extractive green method for the spectrophotometric sequential injection determination of copper(ii) with novel thiazolylazo dyes, RSC Adv. 8 (2018) 15940-15950.
[7] Y. Bazel, M. Lešková, M. Rečlo, J. Šandrejová, A. Simon, M. Fizer, V. Sidey, Structural and spectrophotometric characterization of 2-[4-(dimethylamino)styryl]-1-ethylquinolinium iodide as a reagent for sequential injection determination of tungsten, Spectrochim. Acta, Part A 196 (2018) 398-405.
[8] J. Tóth, Y. Bazeľ, I. Balogh, A fully automated system with an optical immersion probe (OIP) for high-precision spectrophotometric measurements, Talanta 226 (2021) 122185.
[9] J. Škrlíková, V. Andruch, H. Sklenárová, P. Chocholouš, P. Solich, I.S. Balogh, A novel dual-valve sequential injection manifold (DV-SIA) for automated liquid-liquid extraction. Application for the determination of picric acid, Anal. Chim. Acta 666 (2010) 55-61.
[10] J. Škrlíková, V. Andruch, H. Sklenářová, P. Chocholouš, P. Solich, I.S. Balogh, An air-assisted liquid–liquid extraction using a dual-valve sequential injection manifold (DV-SIA): Determination of copper, Anal. Methods 2 (2010) 1134-1139.
[11] C.C. Acebal, H. Sklenářová, J. Škrlíková, I. Šrámková, V. Andruch, I.S. Balogh, P. Solich, Application of DV-SIA manifold for determination of thiocyanate ions in human saliva samples, Talanta 96 (2012) 107-112.
[12] M. Alexovič, M. Wieczorek, J. Kozak, P. Kos̈cielniak, I.S. Balogh, V. Andruch, An automatic, vigorous-injection assisted dispersive liquid-liquid microextraction technique for stopped-flow spectrophotometric detection of boron, Talanta 133 (2015) 127-133.
[13] F. Cacho, J. Masac, R. Zakhar, E. Beinrohr, Indirect electrochemical determination of sulfates in mineral water by a flow-through system, Talanta 207 (2020) 120281.
[14] D. Borosova, A. Manova, J. Mocak, E. Beinrohr, Determination of nickel in hair samples by graphite furnace atomic absorption spectrometry and flow-through stripping chronopotentiometry, Anal. Methods, 2 (2010) 1913-1917.
[15] K. Medinskaia, S. Garmonov, J. Kozak, M. Wieczorek, V. Andruch, P. Kościelniak, A. Bulatov, Stepwise injection determination of isoniazid in human urine samples coupled with generalized calibration method, Microchem. J. 123 (2015) 111-117.
[16] https://www.villalabeco.sk/eng_index.htm, accessed on 30 May 2021.
[17] D. Kaniansky, P. Rajec, A. Švec, P. Havaši, F. Macášek, On-line radiometric detection in capillary isotachophoresis. 1. preliminary experiments, J. Chromatogr. 258 (1983) 238-243.
[18] D. Kaniansky, P. Havaši, J. Marák, R. Sokolík, Post-column amperometric detection in capillary isotachophoresis, J. Chromatogr. 366 (1986) 153-160.
[19] E. Kenndler, D. Kaniansky, Off-line combination of isotachophoresis and mass-spectrometry, J. Chromatogr. 209 (1981) 306-309.
[20] D. Kaniansky, V. Madajová, M. Hutta, I. Žilková, Analysis of asulam in soil by isotachophoresis and liquid chromatography, J. Chromatogr. 286 (1984) 395-406.
[21] J. Hradski, M. Chorváthová Drusková, R. Bodor, M. Sabo, Š. Matejčík, M. Masár, Quantitative aspects of microchip isotachophoresis for high precision determination of main components in pharmaceuticals, Anal. Bioanal. Chem. 408 (2016) 8669-8679.
[22] M. Masár, P. Troška, J. Hradski, I. Talian, Microchip isotachophoresis coupled to surface-enhanced Raman spectroscopy for pharmaceutical analysis, Microchim. Acta 187 (2020) 448.
[23] M. Masár, J. Hradski, M. Nováková, R. Szucs, M. Sabo, Š. Matejčík, Online coupling of microchip electrophoresis with ion mobility spectrometry for direct analysis of complex liquid samples, Sens. Actuators B Chem. 302 (2020) 127183.
[24] J. Piešťanský, M. Matušková, I. Čižmárová, P. Majerová, A. Kováč, P. Mikuš, Ultrasensitive determination of serotonin in human urine by a two dimensional capillary isotachophoresis-capillary zone electrophoresis hyphenated with tandem mass spectrometry, J. Chromatogr. A 1648 (2021) 462190.
[25] http://www.ousa.eu/sk/titulna-stranka-ousa, accessed on 30 May 2021.
[26] https://www.svps.sk/english/, accessed on 30 May 2021.
[27] http://www.biomedcentrum.sav.sk/?lang=en, accessed on 30 May 2021.
[28] https://www.villalabeco.sk/aplikacie.htm, accessed on 30 May 2021.
Flow injection analysis and capillary electrophoresis in Poland
Poland, the largest and most populous country in the Visegrad Group, is today at the forefront of the most rapidly developing countries in Central Europe. Along with the systemic changes related to the collapse of the Soviet Union and gaining sovereignty, Poland began a long-term transformation process towards the west, crowned with joining the structures of NATO and the European Union. This opened up many new opportunities and development paths for Polish science. Along with obtaining EU structural funds for the modernization of scientific facilities, previously unknown opportunities for financing science and developing research infrastructure began to emerge. Over the last dozen years, academic chemical laboratories have been enriched with the most modern equipment, the purchase of which turned out to be beneficial for the development of the scientific potential expressed in publications and patents. Analytical laboratories have gained access to the highest quality spectrometers, chromatographs and other instruments popular today, which guarantee the highest quality of the results of qualitative and quantitative analyzes. Although Poland has not yet caught up with the world's leaders in terms of research infrastructure, it is on the way to it, which allows us to look to the future with optimism and count on new equipment possibilities.
From the very beginning, Flow Injection Analysis (FIA) was also developed by Poles. The creators of FIA (flowinjectiontutorial.com) point to Trojanowicz, Kościelniak, Kojło and Koncki as the most active scientists in the development of flow techniques in a global perspective. Teams, led by these scientists, work to this day, and new ones are formed from their students, ensuring that the continuity of the development of analytical flow techniques will be maintained. Both new original articles on flow injection analysis and inspiring review articles continue to appear. The analysis of the Scopus database results shows that Polish scientists share about 2% of all published in the world articles on flow injection analysis (including sequential flow analysis, multicommutation and paper analytical systems). Over 300 original articles, almost 30 reviews, as well as textbooks, chapters and conference materials are the achievements of just a dozen research centers in Poland. It is worth noting that many of “flow teams” cooperate with each other, which is undoubtedly due to professors: Kościelniak and Trojanowicz, who initiated - firstly national and then international - symposium of flow analysis.
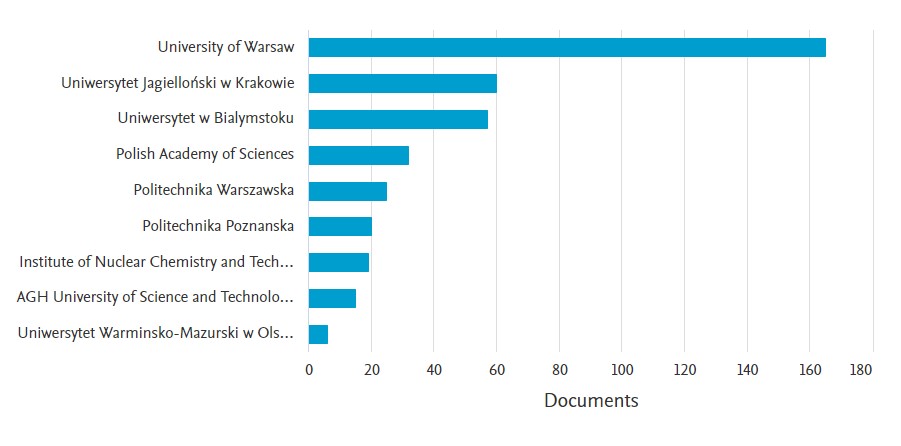
Figure 1. Literature data on the state of different varieties of FIA in Poland according to the Scopus database (May, 2021) received for phrase: ( TITLE-ABS-KEY ( "flow injection analysis" ) OR TITLE-ABS-KEY ( "flow injection" ) OR TITLE-ABS-KEY ( "multicommutated flow" ) OR TITLE-ABS-KEY ( "paper based analytical device" ) OR TITLE-ABS-KEY ( "lab on paper" ) OR TITLE-ABS-KEY ( "sequential injection analysis" ) AND AFFILCOUNTRY ( Poland ) )
Topics undertaken by teams working in Poland cover a wide spectrum of modern flow analytics. Classic flow systems are created, both those that are operated manually and highly automated microprocessor-controlled multicommutated systems. Several of the FIA development teams in Poland propose new construction designs of active or passive flow-through elements new methodologies for acquiring and developing calibration data in flow systems, or the use of rapid prototyping methods to create fluidic systems. Almost all efforts of Polish scientists dealing with the FIA are aimed at demonstrating the practical aspects of the systems they create, therefore the original works contain the results of real analytical scenarios - environmental, clinical or concerning the analysis of food and new materials. The research on paper – based analytical systems for conducting chemical analysis of physiological fluids at the patient's bedside
A separate issue, but still closely related to flow analytical techniques, are microfluidic systems, which are also developed in research teams operating in Poland. In this field, the teams of Garstecki (Institute of Physical Chemistry of the Polish Academy of Sciences), Brzózka (Warsaw University of Technology) and Malechy (Wrocław University of Science and Technology) have the greatest international reputation.
Among the analytical separation techniques, as in the rest of the world, chromatography in its many varieties is the leader. Almost every Polish analytical laboratory, R&D or quality control departments has systems for high performance liquid chromatography (HPLC) and gas chromatography (GC). It is also easier to meet systems coupled with mass spectrometers (HPLC-MS, GC-MS), the numbers of which are already in the hundreds or even higher. As in other countries, capillary electrophoresis (CE) systems, despite numerous advantages over HPLC and GC, are not so popular. Nevertheless, instruments for CE are increasing in Poland from year to year. To the best of the authors' knowledge, their total number in Poland may soon exceed 100, although it will probably always lag behind the much more popular chromatographic systems. For example, in the Department of Analytical Chemistry, Faculty of Chemistry of the Jagiellonian University, there are currently 3 Beckman-Coulter (currently commercially available branded as Sciex) CE systems, including one permanently integrated with MS, a Prince Technologies system, and a lab-built unit developed uder the collaboration with prof. Petr Kuban (IAC, Brno, Czech Republic). Noteworthy, the purchase of the fourth CE system is planned in the very near future. The growing instrumental background in Poland entails a constant progress in the number of publications in which the CE technique played a key role.
According to the Scopus database (as of May 2021), as many as 864 articles with Polish affiliation have been published so far, in which the phrase "capillary electrophoresis" appears in the title and / or summary and / or keywords. The detailed results of the literature analysis are presented in Figure 2. As can be seen in Figure 1A, in recent years there has been a continuous increase in the number of publications that meet the above criteria, which may indicate a growing interest in the CE technique in Poland. This trend begins in the early 1990s, which coincides with the aforementioned socio-political transformation and opening to the west. Since 2002, the number of works on this subject has not fallen below 30 per year, which roughly means that every ten days a new article on CE technology is published, based on studies carried out in Polish research centers. This suggests that the CE technique can by no means be considered a niche, and has been one of the most dynamically developed analytical techniques in Poland for over two decades.
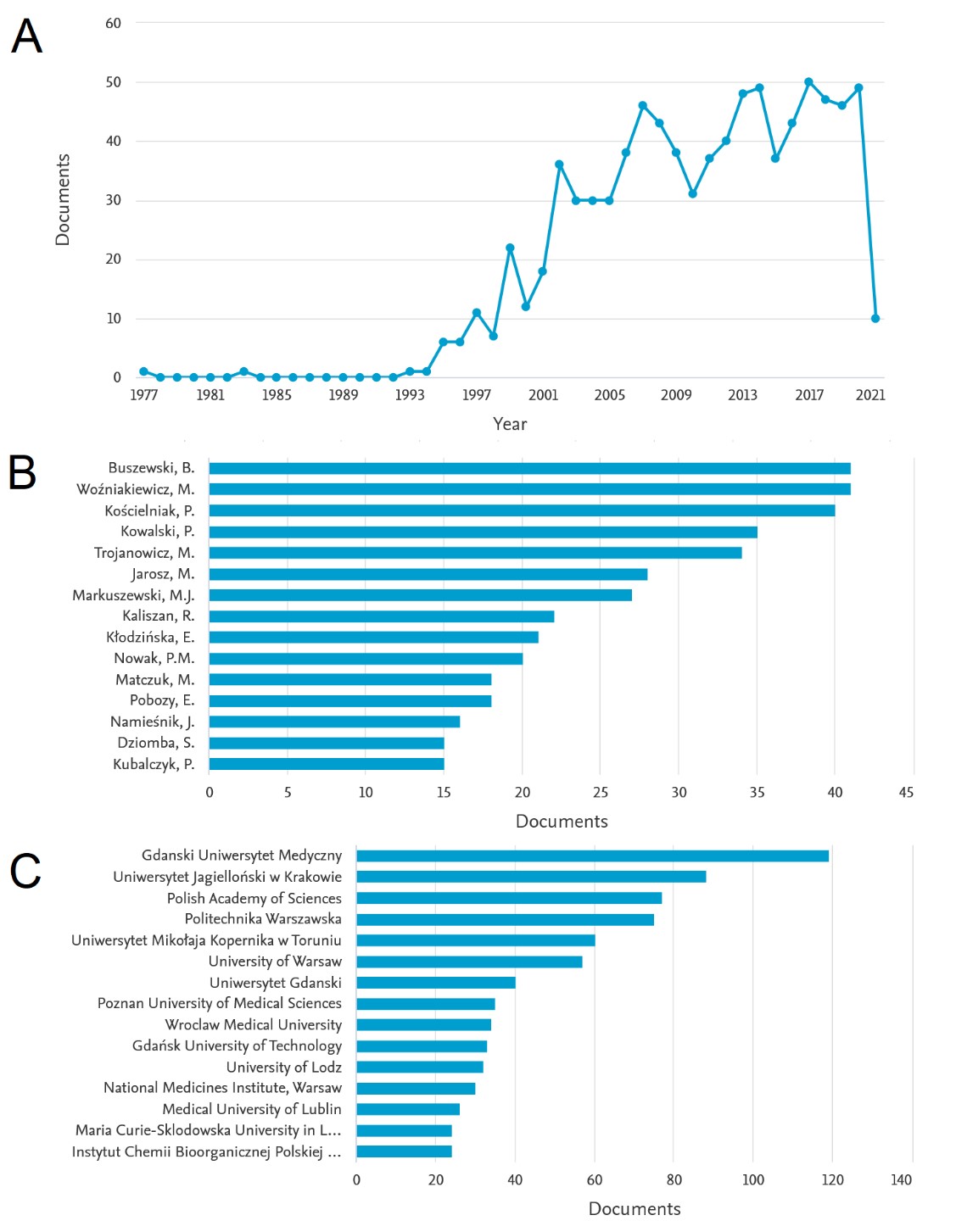
Figure 2. Literature data on the state of CE technology in Poland according to the Scopus database: (A) number of publications on the CE technique in individual years with the indicated Polish affiliation, (B) leading analysts based on the number of publications, (C) leading research centers.
Figures 2B and 2C show the leading scientists associated with the CE technique in Poland and leading research centers. Compared to other countries of the Visegrad Group, Poland has the largest number of large cities with huge academic potential (Kraków, Warsaw, Wrocław, Poznań, Toruń, Łódź, Lublin and others), and most of these cities have at least one CE device.
As the presented analysis shows, the list of authors and centers dealing with the CE technique is long, it is impossible to list all those who actively use it and contribute to its development through numerous publications. In our opinion, however, it is worth mentioning: (i) the group of Professor Bogusław Buszewski from the Nicolaus Copernicus University in Toruń, one of whose numerous achievements in this area is the use of CE systems to separate entire structures of pathogens (viruses and bacteria); (ii) a group of Professor Piotr Kowalski and Professor Michał Markuszewski from the Medical University of Gdańsk, which specialize in the application of CE to generally understood organic and environmental analysis; and (iii) our group from the Jagiellonian University led by Professor Paweł Kościelniak, which uses the CE technique to analyze compounds relevant to forensic and toxicological chemistry.
It is also worth mentioning the involvement of our department in the didactic aspect, expressed by teaching a wide group of students to use CE devices during the implementation of diploma theses, student exercises on analytical panels, and the organization of training and workshops conducted by world-recognized specialists from abroad. An example is the course leading by dr. Cari Sanger van de Griend, collaborating with the Faculty of Chemistry JU within educational projects (2018-2019) and a visiting professorship (2020-2021). In the course the analytical aspects of capillary electrophoresis are elaborated in the background of pharmaceutical and biotechnology industry offering the students to gain specialized skills and knowledge appreciated by Polish industry.
To sum up, the FIA and CE techniques are doing as well in Poland as never before. This is reflected in a long list of scientists representing research centers from many cities and the growing number of articles published in prestigious analytical journals. This state-of-the-art, combined with the constantly tightened national and international cooperation, taking into account, in particular, the dynamically developing cooperation with leading centers from the Visegrad Group countries, allows us to look to the future with optimism and expect new scientific achievements in these fields.
